
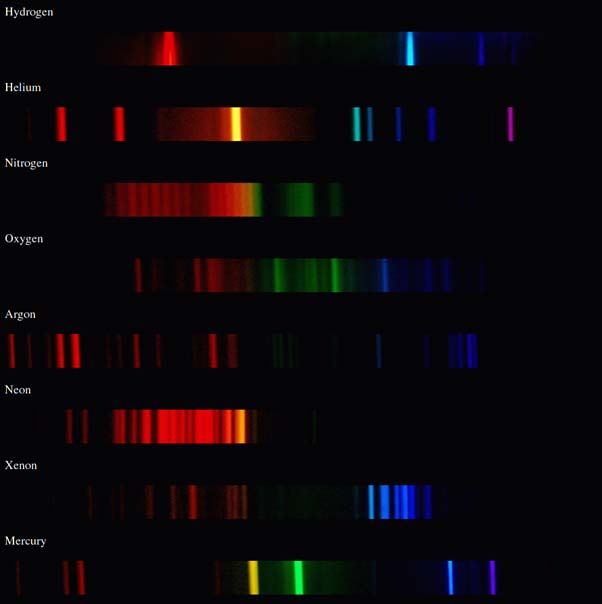

Line up the lines to identify the unknown elements. Is can be used to identify an element in mixtures. So if we can see a spectral line at a given wavelength, it is the result of an electron emitting light as it drops to a lower energy level. Since each element has different numbers of protons, neutrons and most importantly electrons, the emission spectrum for each element is different. You can look at the spectra and identify which elements are present.
#Atomic spectra line spectra series
This series is referred to as the Lyman Series.įurther the electron falls, the more energy it releases. Each element has its own unique atomic emission spectrum. This series is called the Balmer series.Įlectron falls to the 1st energy level, the light given off is t=in the Is in the visible region of the spectrum and spectral line of color are Series of electron transitions is referred to as the Paschen series.Įlectron falls to the 2nd energy principal energy level, the light given off Level, the light given off is in the infrared region of the spectrum. Hydrogen atom, if the electron falls to the 3rd energy principal energy In an emission spectra electrons are excited to an excited state by thermal or electrical means and then relax back to a lower state and emit a photon of light at a specific energy, which is seen as at a specific wavelength. It is when they return to the ground state energy is given off.īut electrons don't have to go directly there. Hydrogen molecules are first broken up into hydrogen atoms (hence the atomic hydrogen emission spectrum) and electrons are then promoted into higher energy. There are two types of line spectra, emission and absorption. Ground state (lowest energy configuration)Įxcited State (higher energy configuration)Ģ-6-1 **Note the # of electrons are the same This information can be useful for plasma diagnostics. spectra of light emission using a diffraction grating. For some spectra, there is also graphical information on dependences of certain line intensity ratios on electron density and/or temperature in the emitting plasma. lines in the solar spectrum millions of such fixed absorption lines are now known. A line spectrum is like a fingerprint - it can be used to identify the element. Different elements produce different line spectra. This is not the cause of the spectral lines. Graphical output is available in the forms of Grotrian diagrams, line identification plots, and Saha-LTE spectrum plots. Line emission spectra are unique to a particular element. "Excite electrons fall back to lower energy levels releasing energy in the form of light."Įxciting an electron promotes it to a higher sublevel of energy level. Δ E Δ t ⪆ ℏ, can also be used, when the signal-to-noise ratio in the spectrum is sufficiently high.Lines Spectra and Excited Electron States According to the uncertainty principle the uncertainty in energy, Δ E and the lifetime, Δ t, of the excited state are related by Drill down to an element and inspect the individual spectra lines. Sort by name, symbol, or count of spectra lines. Can search by element name, symbol, or atomic number.
#Atomic spectra line spectra tv
Broadening can only be mitigated by the use of specialized techniques, such as Lamb dip spectroscopy. Mac iPhone iPad Apple TV Reference app for Strong Line Atomic Emission Spectra in the visible range of 380-740nm. What you see is determined by environment. Numerous factors can contribute to the broadening of spectral lines. Absorption and emission spectral lines are at exactly the same wavelengths. However, when this energy is measured by means of some spectroscopic technique, the line is not infinitely sharp, but has a particular shape. A display, like those you say in the lab, of the different colors is called an emission spectrum of the element. The spectrum consists of a series of overlapping lines belonging to a vibronic progressionĪn atomic transition is associated with a specific amount of energy, E. Absorption spectrum of an aqueous solution of potassium permanganate. Emission spectra For this relationship: As the energy levels have different values, each of the possible electron transitions within an atom will produce a.


 0 kommentar(er)
0 kommentar(er)
